Research
I am an applied mathematician specializing in the use of mathematical tools to investigate biological problems. My primary line of research focuses in understanding the intricate relationship between immunometabolism and its implications in infectious diseases and inflammation using applied analysis and dynamical systems. More generally, I am interested in developing data-driven mechanistic models for understanding the underlying biology of specific biological processes including the dynamics of infectious diseases in populations and in hosts. When the complexity of the model allows it, I use dynamical systems theory and applied analysis techniques including parameter bifurcation to extract important information about the problem. In most cases however, these models cannot be solved analytically and it is necessary the use of existent or own written numerical packages. Analytic or numerical analysis of the model can reveal rich insights about the biology of the problem that would allow us to address relevant biological questions as well as generate and test new hypothesis. I am also interested in using statistical methods and machine learning algorithms to understand data patterns in clinical or experimental data, which can later inform the development of mathematical models to comprehend underlying biological mechanisms and generate/test hypotheses to be validated through further experiments.
Projects
Mathematical Modeling of Energy Consumption in the Acute Inflammatory Response.
- Several studies have found a relation between sepsis and low levels of adenosine triphosphate (ATP) combined with overproduction of lactate and nitric oxide (NO). In this project we developed a system of ordinary differential equations to study the dynamics of the acute inflammatory response and its interactions with the production and demand of ATP. We also explored some altered metabolic states such as hypoglycemia, hyperglycemia, and hypoxia in the presence of sepsis obtaining consistent resuts with the literture.
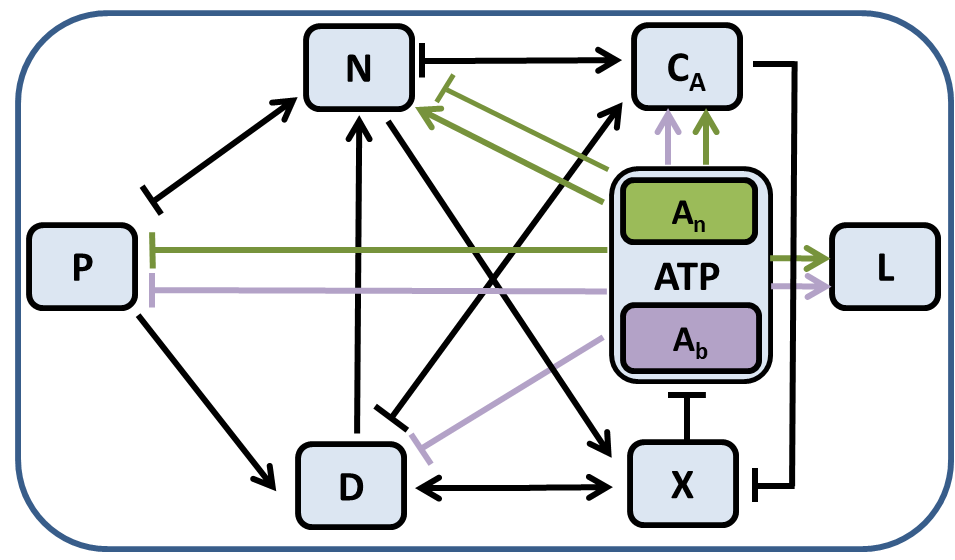
.png)
(Left) Interactions between the eight variables of the system of equations in the model. P corresponds to pathogens, D tissue damage, N phagocytes, CA anti-inflammatory mediators, X nitric oxide, L lactate, and ATP adenosine triphosphate. (Right) Bifurcation diagram for model equations on. Equilibrium states are illustrated with red solid curves (stable), shown with dashed curves (unstable), or not shown at all if non-physiological. The three stable equilibrium states correspond to healthy, aseptic (infection is resolved but inflammation persists), and septic (infection and inflammation persist) states.
Using our model we generated a set of virtual patients by introducing variability to sensitive parameters. Simulating the time courses of their inflammatory responses until reaching one of the model equilibrium states, healthy, aseptic, or septic and defining the time of death as the time at which tissue damage reaches certain threshold we classified patients between survivors and no survivors.
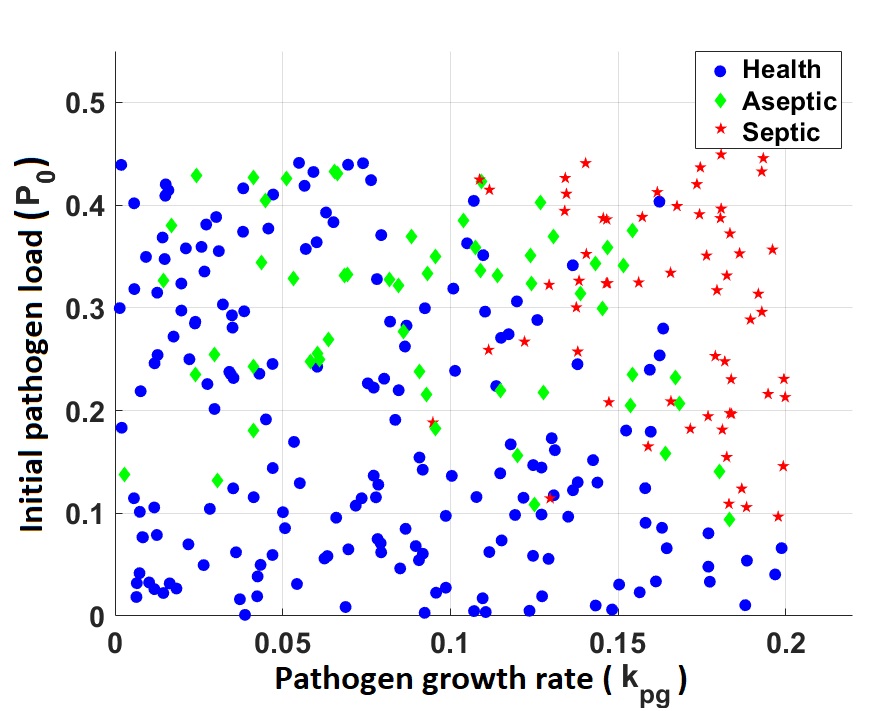
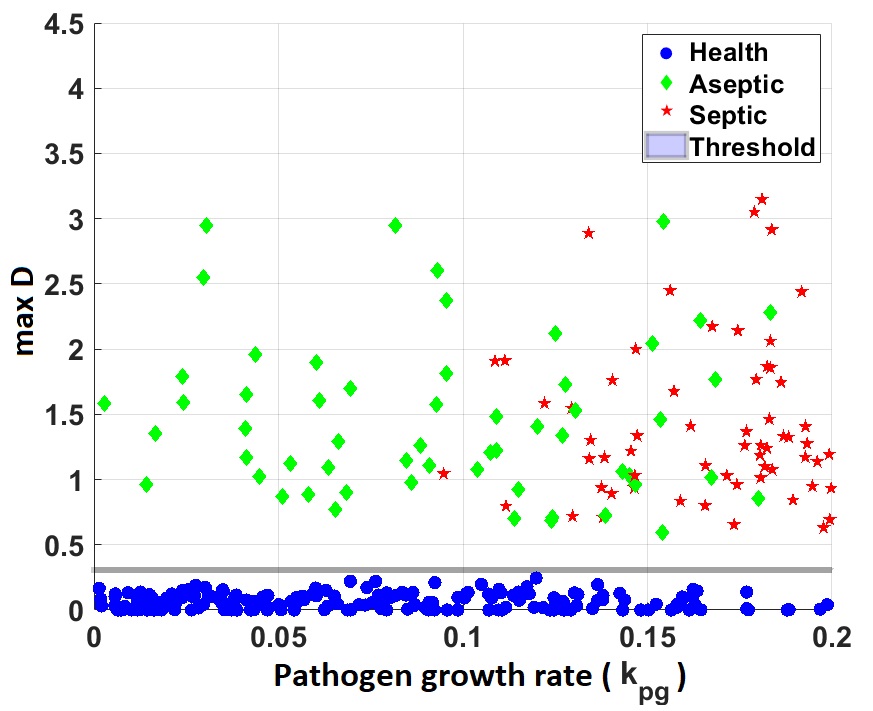
Representation of virtual subjects projected on the initial pathogen load ($P_0$) - pathogen growth rate ($k_{pg}$) plane (left) and projected on the $P_0$ - maximum damage vaule attained over the course of patient simulation (right).
A Data-driven Mathematical Study of the Role of Energy in Sepsis.
- In a subsequent publication we further extended our model to include quantities that were measured from the blood of experimental subjects exposed to bacteria. By fitting model dynamics to experimental data, we derived parameter distributions corresponding to individual subjects’ responses as well as a parameter ensemble representing the full cohort of experimental subjects. We found important differences across survivors and non-survivors and identified the role of energetics in each of the groups and determined the possible causes of death of the non-survivor cohort.
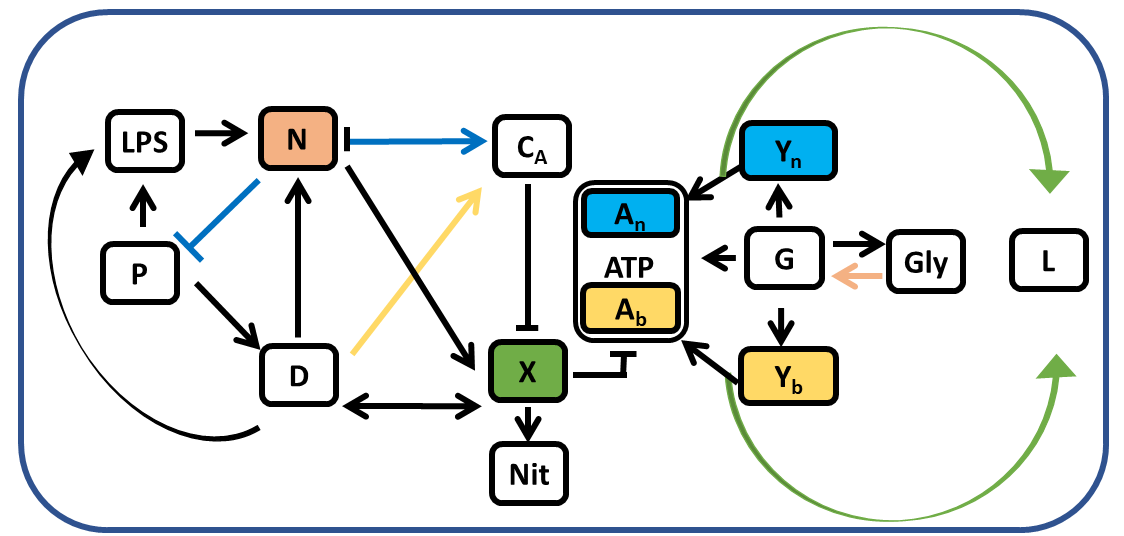
Interactions between the 12 variables of the system of equations in the model. P corresponds to pathogens, D tissue damage, N phagocytes, CA anti-inflammatory mediators, X nitric oxide, L lactate, ATP adenosine triphosphate, LPS lipopolysachiride, Nit nitrate, G glucose, Y pyruvate, and Gly glycogen.
.jpg)
.jpg)
Predicted intervals of the ATP and phagocytes model trajectories obtained with parameters from survivor (blue) and non-survivor (red) distributions. The model suggested survivor individuals were able to recover their ATP loss during the infection, whereas in non-survivors energy depletion was a potential reason for death. Similarly, phagocyte activity remained elevated in non-survivors while baseline levels were attained in the survivor cohort
